| [Previous
Story] [Next
Story]
STM ELUCIDATES MECHANISM
Molecule-at-a-time study reveals fundamentals of classic
reaction
MITCH JACOBY
Want to validate a
reaction mechanism? Why not prepare the reaction intermediate, trap
it, take a picture of it, and then watch it react--one molecule at
time? Researchers in California have taken this approach to study
the oxidation of carbon monoxide to carbon dioxide.
One of the simplest and most well-studied reactions,
oxidation of CO to CO2 stands as an archetypal reaction
in heterogeneous catalysis. But more than a model system, the
elementary reaction is central to automobile emissions control, air
purification, and chemical sensing.
For decades, surface scientists have used nearly
every tool in their bags to get a handle on the reaction mechanism.
Yet a clear picture of the fundamental steps remained
elusive.
Now, using a scanning tunneling microscope (STM)
with unique capabilities, University of California, Irvine, physics
and chemistry professor Wilson Ho and postdoctoral associate Jae Ryang Hahn
have synthesized and studied an O-CO-O complex. And they've
identified the previously proposed but unobserved surface species as
an intermediate in the CO oxidation reaction [Phys. Rev. Lett.,
87, 166102
(2001)].
To date, only a few groups--among them Ho's and that
of Karl-Heinz Rieder, a professor of physics at Free University in
Berlin--have reported examples of step-by-step synthesis using an
STM. According to Rieder, the present study is "important work in
the field of single-molecule engineering due to the model character
of the reaction."
Taking advantage of the single-molecule dexterity
for which scanning probe instruments have become famous, Ho and Hahn
use an STM tip to gently coax a CO molecule toward a pair of closely
spaced oxygen atoms on a frigid silver surface. Very low
temperatures minimize interfering vibrations and help confine
reactants and intermediates to the surface. As the molecule is moved
to within 2 Å of the oxygen atoms, the atoms and molecule form an
O-CO-O complex. By applying a brief electron pulse to the complex,
the team energizes the species and causes it to react. From its
excited state, O-CO-O goes on to form CO2, which desorbs
from the surface, leaving behind a lone oxygen atom.
Identifying a second reaction pathway, the Irvine
group shows that CO2 can be formed by depositing a CO
molecule from an STM tip onto an isolated oxygen atom.
Ordinarily, scanning probe techniques cannot provide
chemical information. But in recent years, Ho and his associates
worked to overcome that limitation by pioneering procedures by which
an STM can be used to measure highly sensitive vibrational data.
Vibrational analysis of O-CO-O plays a central role in the present
study.
"Not only does the STM allow us to make the complex,
but it allows us to image it and characterize it spectroscopically
via single-molecule vibrational spectroscopy and microscopy," Ho
says.
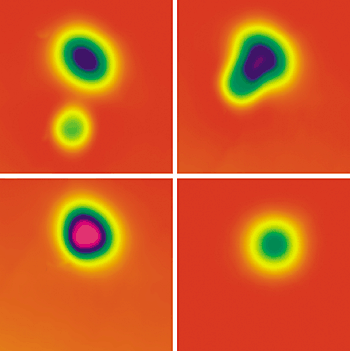
NUDGE, NUDGE! A pair of
oxygen atoms (oblong) and a CO molecule (circular) appear as
distinct features in an STM image (top left). As the species are
brought closer together (top right), the image changes until, at
less than 2 Å separation, an O–CO–O complex forms (bottom left).
Exciting the complex causes CO2 to form and desorb,
leaving behind a lone O atom (bottom right).
[Previous
Story] [Next
Story]
Chemical & Engineering News
Copyright ©
2001 American Chemical Society | 
