

Concept
Boiling occurs at the temperature at which the liquid’s vapor pressure equals the surrounding environmental pressure. Under standard conditions of pressure (one atmosphere) and elevation (sea level), water boils at 100 °C.
Since the boiling point decreases with decreasing vapor pressure, the pressure inside of the flask and surrounding the water can be lowered with a vacuum pump until the water vigorously boils at room temperature, ~ 22 °C. These concepts explain why cooking at higher elevation, where the atmospheric pressure is reduced, requires greater cooking times.
Procedure
- Verify that the flask is filled to the 1000 ml mark with water and that the vacuum pump is plugged into the flask.
- If desired, place the flask on the front edge of an overhead projector and turn the projector on.
- Securely plug the mouth of the flask with the rubber stopper.
- Turn on the vacuum pump using the toggle switch located on the back of the pump near the power cable.
- Notice that the water boils after about 30 seconds.
- Turn off the vacuum pump and gently uncork the flask to equalize the pressure.
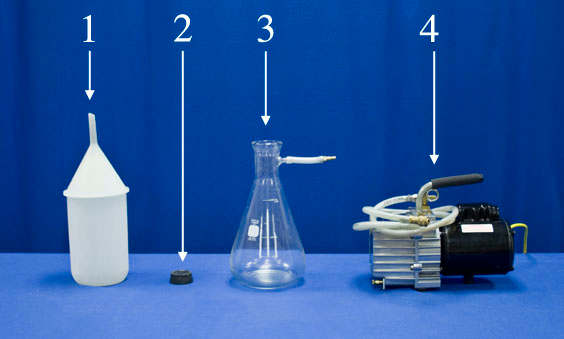
Equipment
- Water and Funnel
- Large Rubber Stopper
- 4000 ml Flask with Air Valve
- Vacuum Pump
- Overhead Projector (not
- shown)