

Concept
At equilibrium, the average number of balls per volume should be roughly the same through out the container. Since the above container is rectangular with fixed thickness, the ratio of the number of balls in the larger region to the number of balls in the smaller region should be roughly equal to the ratio of the larger region’s width to the smaller region’s width. In the above photo, this ratio is about 3:1.
Procedure
- Place the small balls on one side of the divider attachment in the demonstrator’s field and fully extend the adjustable feet to tilt the demonstrator as much as possible.
- Turn on the overhead projector and toggle the power switch on the side of the demonstrator to turn it on.
- Slowly rotate the black knob clockwise to adjust the speed of the agitator bars to full power.
- Wait about 10 seconds for the balls to reach equilibrium.
- Stop the agitator bars and count the number of balls on each side of the divider attachment.
- Notice that the number of balls on each side of the divider attachment is proportional to the area on each side of the attachment.
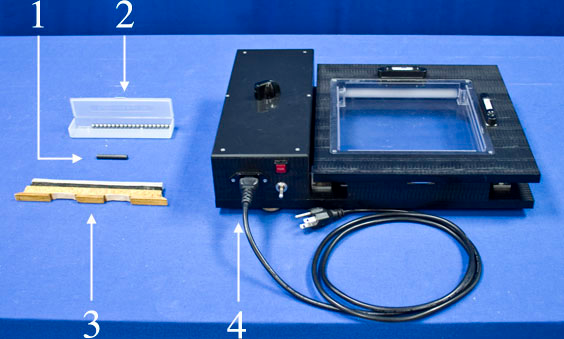
Equipment
- Box of 10 Small Balls, 10 Medium Balls and 3 Large Balls
- Divider Attachment
- Small Magnet
- Molecular Motion Demonstrator
- Overhead Projector (not pictured)