
Concept
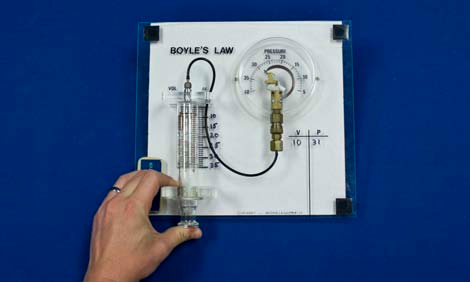
Here is a straightforward demonstration of the inverse relationship between pressure $P$ and volume $V$ as dictated by the ideal gas law with temperature held constant in a closed system. As the volume of a syringe is increased, the audience can clearly see the pressure gauge values decrease. These values are recorded and a quick plot of $P$ versus $\frac{1}{V}$ will reveal the predicted relation.
Procedure
- Place the Boyle’s Law Apparatus on the overhead projector or document camera.
- Notice that the hand-written volume markings should be used for all the measurements (see Notes).
- Begin with the end of the plunger at the 20 cc mark and the pressure gauge at about 15 lbs/in2 (atmospheric pressure).
- Push the plunger to the 10 cc mark and record the corresponding pressure reading on the Plexiglas board using the provided marker.
- Continue recording pressure readings for the 15, 20, 25 and 30 cc marks.
Equipment
- Boyle’s Law Apparatus
- Transparency Marker
- Overhead Projector or
- Document Camera (not pictured)