![]()
![]()
![]()
 Binary liquid mixtures
are liquids that are composed of two different molecular components, A
and B. For a particular range of temperatures and concentrations a typical
binary liquid mixture will phase separate into two different liquid phases,
like a mixture of oil and water. We will denote the two liquid phases as
Binary liquid mixtures
are liquids that are composed of two different molecular components, A
and B. For a particular range of temperatures and concentrations a typical
binary liquid mixture will phase separate into two different liquid phases,
like a mixture of oil and water. We will denote the two liquid phases as
![]() (rich in the A component) and
(rich in the A component) and
![]() (rich in the B component).
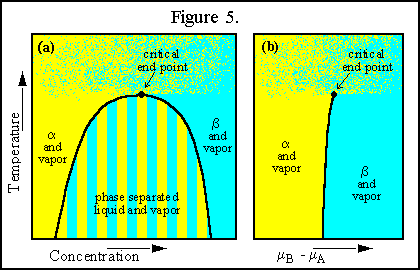
A phase diagram for a phase separating binary liquid mixture coexisting
with its vapor is shown in Figure 5 in both temperature-concentration space
(a) and temperature-chemical potential space (b). The region of three coexisting
phases (
(rich in the B component).
A phase diagram for a phase separating binary liquid mixture coexisting
with its vapor is shown in Figure 5 in both temperature-concentration space
(a) and temperature-chemical potential space (b). The region of three coexisting
phases (![]() ,
, ![]() and
vapor) of Figure 5(a) becomes a line when plotted as in Figure 5(b) and
will therefore be referred to as the triple line.
and
vapor) of Figure 5(a) becomes a line when plotted as in Figure 5(b) and
will therefore be referred to as the triple line.
A triple line can be considered as a line of triple points. Consequently,
as was shown by Pettersen and Saam [30],
in a binary liquid system there can be a line of triple point induced dewetting
transitions-- a dewetting line. This will occur in systems in which both
![]() and
and ![]() separately
wet the interface between a substrate and the vapor, but one liquid, say
separately
wet the interface between a substrate and the vapor, but one liquid, say
![]() , wets more strongly
than the other (assuming that
, wets more strongly
than the other (assuming that ![]() does
not wet the interface between
does
not wet the interface between ![]() and
the vapor). Furthermore, if there is a transition on the triple line between
wetting films (on the substrate) of
and
the vapor). Furthermore, if there is a transition on the triple line between
wetting films (on the substrate) of ![]() and
films of
and
films of ![]() , then there
will be a dewetting line attached to that transition.
, then there
will be a dewetting line attached to that transition.
If ![]() wets the interface
between
wets the interface
between ![]() and the vapor, then
and the vapor, then ![]() films
become
films
become ![]() -
-![]() films. In this case, a transition between the two different wetting films,
films. In this case, a transition between the two different wetting films,
![]() and
and ![]() -
-![]() ,
is equivalent to a wetting transition of
,
is equivalent to a wetting transition of ![]() at
the interface between the substrate and
at
the interface between the substrate and ![]() .
Such a wetting transition will have both prewetting and dewetting lines.
This situation is illustrated in the temperature-chemical potential phase
diagram of Figure 6.
.
Such a wetting transition will have both prewetting and dewetting lines.
This situation is illustrated in the temperature-chemical potential phase
diagram of Figure 6.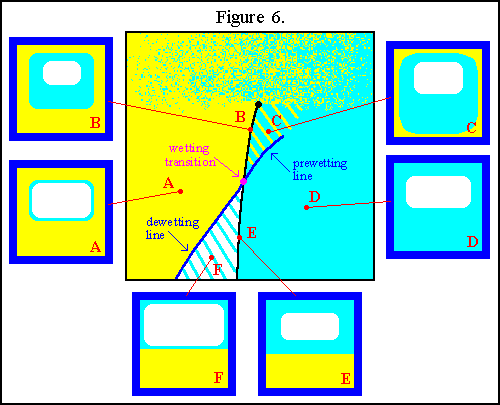 The six boxes to either side of and below the phase diagram illustrate
the adsorption states for the case that the substrate forms the walls of
a container. At point A, there is bulk
The six boxes to either side of and below the phase diagram illustrate
the adsorption states for the case that the substrate forms the walls of
a container. At point A, there is bulk
![]() and vapor in the container with
a thin layer of
and vapor in the container with
a thin layer of ![]() between
them, and
between
them, and ![]() films wet the walls
of the container. Moving towards point B,
on the triple line above the wetting transition, the
films wet the walls
of the container. Moving towards point B,
on the triple line above the wetting transition, the ![]() layer
diverges in thickness so that all three bulk phases are present in the
container. Proceeding to point C, the
layer
diverges in thickness so that all three bulk phases are present in the
container. Proceeding to point C, the
![]() phase is no longer stable in the
bulk so the
phase is no longer stable in the
bulk so the ![]() films become microscopic
in thickness. The substrate is still wet, however, because of the thick
films become microscopic
in thickness. The substrate is still wet, however, because of the thick
![]() films. In crossing
the prewetting line towards point D,
the thin
films. In crossing
the prewetting line towards point D,
the thin ![]() films disappear altogether
leaving only the
films disappear altogether
leaving only the ![]() phase
to wet the container walls. At point E,
on the triple line below the wetting transition, there is again both bulk
phase
to wet the container walls. At point E,
on the triple line below the wetting transition, there is again both bulk
![]() and
and ![]() in
the container, but only
in
the container, but only ![]() wets
the walls. If the system is shifted towards point F,
the
wets
the walls. If the system is shifted towards point F,
the ![]() films remain
on the walls, but
films remain
on the walls, but ![]() is
no longer stable in the bulk. So in the region around point F,
the walls of the container are non-wet.
is
no longer stable in the bulk. So in the region around point F,
the walls of the container are non-wet.
![]()
![]()
![]()